Please refer to the MCQ Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations with Answers. The following Chemical Reactions and Equations Class 10 Science MCQ Questions have been designed based on the current academic year syllabus and examination guidelines for Class 10. Our faculty has designed MCQ Questions for Class 10 Science with Answers for all chapters as per your NCERT Class 10 Science book.
Chemical Reactions and Equations Class 10 MCQ Questions with Answers
Please see below Chemical Reactions and Equations Class 10 Science MCQ Questions, solve the questions and compare your answers with the solutions provided below.
Question: Which of the following statements about the given reaction are correct?
3Fe (s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent
(a) (i), (zi) and (iii)
(b) (in) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer
C
Question: Magnesium ribbon is rubbed before burning because it has a coating of
(a) basic magnesium carbonate
(b) basic magnesium oxide
(c) basic magnesium sulphide
(d) basic magnesium chloride
Answer
A
Question: Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
A
Question: The process of reduction involves
(a) addition of oxygen
(b) addition of hydrogen
(c) removal of oxygen
(d) removal of hydrogen
Answer
B
Question: Three beakers labelled as A, B and C each containing 25 ml of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solution contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is (are) correct?
(i) In beakers A and B, exothermic process has occurred.
(ii) In beakers A and B, endothermic process has occuBftd.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (iv), (ii) and (iii)
Answer
C
Question: Oxidation is a process which involves
(a) addition of oxygen
(b) addition of hydrogen
(c) removal of oxygen
(d) removal of hydrogen
Answer
A
Question: Give the ratio in which hydrogen and oxygen are present in water by volume.
(a) 1:2
(b) 1:1
(c) 2:1
(d) 1:8
Answer
A
Question: MnO2 + 4HCl → 2 + 2H2O + Cl2
Identify the substance oxidized in the above . equation.
(a) MnCl2
(b) HCl
(c) H2O
(d) MnO2
Answer
D
Question: Which of the following is an endothermic process?
(a) Dilution of sulphuric acid
(b) Sublimation of dry ice
(c) Condensation of water vapours
(d) Respiration in human beings
Answer
B
Question: When Ag is exposed to air it gets a black coating of
(a) AgNO3
(b) Ag2S
(c) Ag2O
(d) Ag2CO3
Answer
B
Question: A redox reaction is one in which –
(a) both the substances are reduced.
(b) both the substances are oxidised.
(c) an acid is neutralised by the base.
(d) one substance is oxidised while the other is reduced.
Answer
D
Question: A substance which oxidises itself and reduces other is known as –
(a) oxidising agent
(b) reducing agent
(c) both of these
(d) none of these
Answer
B
Question: In the equation, NaOH + HNO3 → NaNO3 + H2O
nitric acid is acting as –
(a) an oxidising agent
(b) an acid
(c) a nitrating agent
(d) a dehydrating agent
Answer
B
Question: Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a –
(a) combination reaction
(b) double displacement reaction
(c) decomposition reaction
(d) displacement reaction
Answer
D
Question: In the following equations :
Na2CO3 + x HCl → 2 NaCl + CO2 + H2O, the value of x is–
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question: White silver chloride in sunlight turns to –
(a) grey
(b) yellow
(c) remain white
(d) red
Answer
A
Question: When copper powder is heated it gets coated with –
(a) black copper oxide
(b) yellow copper oxide
(c) red copper oxide
(d) None of these
Answer
A
Question. When ferrous sulphate is heated strongly it undergoes decomposition to form ferric oxide as a main product accompanied by a change in colour from:
(a) Blue to green.
(b) Green to blue.
(c) Green to brown.
(d) Green to yellow.
Answer
C
Question. Which of the following is termed as oxidizing agent?
(a) Which gives Oxygen
(b) Which removes oxygen
(c) Which gives hydrogen
(d) All of the above
Answer
A
Question. Zn2+ (aq) +2e -> Zn(s). this is
(a) Oxidation
(b) Reduction
(c) Redox reaction
(d) None of these
Answer
B
Question. Formula of magnesium oxide.
(a) MnO2
(b) MgO
(c) Mg2O
(d) MnO
Answer
B
Question. A sample of copper carbonate when heated in a glass tube it forms a black residue with the evolution of colourless gas. Identify the gas ?
(a) Carbon Dioxide
(b) Hydrogen
(c) Nitrogen
(d) Sulphur
Answer
A
Question. We store silver chloride in a dark coloured bottle because :
(a) Undergoes redox reaction
(b) It is a white solid
(c) To avoid action by sunlight
(d) None of these
Answer
C
Question. Burning of natural gas……….. choose a product.
(a) CO2 , H2O
(b) methane and water
(c) ethane and carbondioxide
(d) carbondioxide and oxygen
Answer
A
Question. The carbon dioxide gas is passed through a lime water, which is chemical change due to formation of _______.
(a) Greyish colour
(b) Greenish colour
(c) Milky colour
(d) Brownish colour
Answer
C
Question: Combination of phosphorus and oxygen is an example of–
(a) oxidation
(b) reduction
(c) rancidity
(d) None of these
Answer
A
Question: Black and white photography uses –
(a) decomposition of silver chloride.
(b) decomposition of silver bromide.
(c) both
(d) none of these
Answer
B
Question: Rusting of iron is an example of –
(a) reduction
(b) redox
(c) oxidation
(d) dissociation
Answer
B
Question: Take about 1.0g CaCO3 in a test tube. Heat it over a flame, a colourless gas comes out. The reaction is called a
(a) decomposition reaction
(b) displacement reaction
(c) double decomposition reaction
(d) double displacement reaction
Answer
A
Question: Hydrogen sulphide (H2S) is a strong reducing agent. Which of the following reactions shows its reducing action?
(a) Cd(NO3)2 + H2S → CdS + + 2HNO3
(b) CuSO4 + H2S → CuS + H2SO4
(c) 2FeCl3 + H2S→ 2FeCl2 + 2HCl + S
(d) Pb(NO3)2 + H2S → PbS + 2CH3COOH
Answer
C
Question: Which of the following does not corrode when exposed to the atmosphere?
(a) Iron
(b) Copper
(c) Gold
(d) Silver
Answer
C
Question: Match chemical reactions given in the List I with the type of chemical reactions given in List II and select the correct answer using the options given below:

(a) A-I, B-V, C-III, D-IV
(b) A-III, B-IV, C-V, D-I
(c) A-IV, B-III, C-V, D-I
(d) A-III, B-I, C-II, D-IV
Answer
D
Question: When copper turnings are added to silver nitrate solution,a blue coloured solution is formed after some time. It is because, copper –
(a) displaces silver from the solution
(b) forms a blue coloured complex with AgNO3
(c) is oxidised to Cu2+
(d) is reduced to Cu2+
Answer
A
Question: Zn2+(aq) + 2e– → Zn(s). This is –
(a) oxidation
(b) reduction
(c) redox reaction
(d) none of these
Answer
B
Question: A substance A reacts with another substance B to produce the product C and a gas D. If a mixture of the gas D and ammonia is passed through an aqueous solution of C, baking soda is formed. The substances A and B are
(a) HCl and NaOH
(b) HCl and Na2CO3
(c) Na and HCl
(d) Na2CO3 and H2O
Answer
B
Question: 2CuI → Cu + CuI2, the reaction is –
(a) redox
(b) neutralisation
(c) oxidation
(d) reduction
Answer
A
Question: The oxidation number of sulphur is –4 in
(a) H2S
(b) CS2
(c) Na2SO4
(d) Na2SO3
Answer
D
Question: Identify the endothermic process from the following
(a) Addition of conc. HCl to water
(b) CH4(g) +2O2(g) → CO2(g) + 2H2O(1)
(c) H2O(1)→ H2O(g)
(d) CaO(s) + H2O(1) → Ca(OH)2(aq)
Answer
C
Question: The oxidation states of P atom in POCl3, H2PO3 and H2P2O6, respectively are
(a) + 5, + 4, + 4
(b) + 5, + 5, + 4
(c) + 4, + 4, + 5
(d) + 3, + 4, + 5
Answer
A
Question: What is the chemical name for slaked lime ?
(a) Sodium hydroxide
(b) Calcium oxide
(c) Calcium hydroxide
(d) Sodium chloride
Answer
C
Question: Which of the following metal is protected by a layer of its oxide ?
(a) Copper
(b) Iron
(c) Aluminium
(d) Sodium
Answer
C
Question: In which of the following, the identity of initial substance remains unchanged ?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer
B
Question: The reaction between calcium oxide and water is :
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) Double-decomposition reaction
Answer
A
Question: On the basis of evolution or absorption of heat, chemical reactions can be divided in how many types ?
(a) Two
(b) Three
(c) Four
(d) One
Answer
A
Question: Heating of ferrous sulphate is a type of :
(a) Decomposition reaction
(b) Combination reaction
(c) Displacement reaction
(d) All of the above
Answer
A
Question: To balance Al(OH)3 + HNO3 → Al(NO3)3 + H2O number of HNO3 molecules required will be :
(a) 2
(b) 4
(c) 3
(d) 8
Answer
C
Question: The oxidation reaction which produces heat and light is called :
(a) Endothermic
(b) Photochemical
(c) Exothermic
(d) Combustion
Answer
D
Question: Which metal is displaced when lead is put in the solution of copper chloride ?
(a) Lead
(b) Copper
(c) Chlorine
(d) All of the above
Answer
B
Question: The colour of ferrous sulphate crystals is :
(a) Deep green
(b) Light green
(c) Bluish green
(d) None of the above
Answer
B
Question: Chemically the ‘water gas’ is
(a) H2O (gaseous)
(b) CO2 + H2
(c) CH4 + H2O
(d) CO + H2
Answer
D
Question: Silver articles become black when exposed to air. It is due
to the formation of
(a) Silver oxide
(b) Silver nitrate
(c) Silver chloride
(d) Silve sulphide
Answer
D
Question: A test tube along with calcium carbonate in it initially weighed 30.08 g. A heating experiment was performed on this test tube till calcium carbonate completelydecomposed with evolution of a gas. Loss of weight during this experiment was 4.40 g. What is the weight of the empty test tube in this experiment?
(a) 20.08 g
(b) 21.00 g
(c) 24.50 g
(d) 2.008 g
Answer
A
Question: The process of respiration is :
(a) Oxidation reaction which is endothermic
(b) Reduction reaction which is endothermic
(c) Combination reaction which is exothermic
(d) Oxidation reaction which is exothermic
Answer
D
Question:

If we added FeSO4 to above four test tubes, in which test tube we observe black residue?
(a) “A” and “B”
(b) “B” and “C”
(c) “A” and “C”
(d) “B” and “D”
Answer
D
Question. CuSO4 + Zn → Cu + ZnSO4 This reaction is an example of a:
(a) Combination reaction.
(b) Double displacement reaction.
(c) Decomposition reaction.
(d) Displacement reaction.
Answer
d
Question. Write the balanced reaction of Calcium oxide with water and state what type of reaction is this
(a) CaO + H2O→ CaOH + H2, displacement
(b) CaO + H2O → Ca(OH)2, combination
(c) CaO + H2O → Ca(OH)2, decomposition
(d) CaO + H2O→ CaOH, combination
Answer
B
Question. Identify the substances that are oxidised and the substances that are reduced in the following reactions. CuO(s) + H2(g) → Cu(s) + H2O(l)
(a) Cu is oxidised, H2O is reduced
(b) CuO is oxidised, H2O is reduced
(c) H2 is oxidised, CuO is reduced
(d) H2 is oxidised, H2O is reduced
Answer
C
Question. A solution of a substance ‘X’ is used for white washing. Name the substance ‘X’ and write its formula.
(a) Lime stone, CaCO3
(b) Lime , CaCO3
(c) Calcium oxide , CaO
(d) Calcium carbonate , CaCO3
Answer
C
Question. Identify the type of reaction in each case. Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g) Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
(a) Combination, Decomposition
(b) Double displacement, Combination
(c) Decomposition, Combination
(d) Displacement, Decomposition
Answer
C
Question. The balancing of chemical equations is in accordance with:
(a) Law of combining volumes
(b) Law of constant proportions
(c) Law of conservation of mass
(d) Both b and c
Answer
C
Question. What type of reaction is respiration
(a) Exothermic
(b) Endothermic
(c) Reduction reaction
(d) Combination reaction
Answer
A
Question. Which of the statements about the reaction below are incorrect? Fe2O3(s) + 3CO(g) → 2Fe(s) +3CO2(g)
(a) Iron is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon monoxide is getting oxidised.
(d) Iron oxide is getting reduced.
i. a&b
ii. a & c
iii. c & d
iv. all
Answer
C
Question. What happens when dilute Sulphuric acid is added to Zn granules? Tick the correct answer.
(a) Hydrogen gas and Zinc chloride are produced.
(b) Chlorine gas and Zinc hydroxide are produced.
(c) No reaction takes place.
(d) Zinc salt and water are produced.
Answer
A
Question. What amount of CO2 is evolved when 50g of CaCO3 is strongly heated ?
(a) 0.25 mol
(b) 0.35 mol
(c) 0.45 mol
(d) 0.5 mol
Answer
4
Question. In the equation, NaOH + HNO3 →NaNO3 + H2Onitric acid is acting as
(a) an oxidising agent
(b) an acid
(c) a reducing agent
(d) a dehydrating agent
Answer
2
Question. When lead nitrate reacts with potassium iodide, yellow precipitate of
(a) PbI2 is formed
(b) KNO3 is formed
(c) Pb(NO3)2 is formed
(d) PbIO3 is formed
Answer
1
Question. Among the following metals, which corrodes negligibly ?
(a) Gold
(b) Platinium
(c) Palladium
(d) All of the above
Answer
4
Question. What happens when copper rod is dipped in ironsulphate solution ?
(a) Copper displaces iron.
(b) Blue colour of copper sulphate solution is obtained.
(c) No reaction takes place.
(d) Reaction is exothermic.
Answer
3
Question. A student added dilute HCl to a test tube containing zinc granules and made following observations :
I. The zinc surface became dull and black.
II. A gas evolved which burnt with a pop sound.
III. The solution remained colourless.
Correct observations are
(a) I and II
(b) I and III
(c) II and III
(d) I, II and III
Answer
4
Question. A dilute solution of sodium carbonate was added to two test tubes-one containing dil HCl (A) and the other containing dilute NaOH (B). The correct observation was
(a) A brown coloured gas liberated in test tube A.
(b) A brown coloured gas liberated in test tube B.
(c) A colourless gas liberated in test tube A.
(d) A colourless gas liberated in test tube B.
Answer
3
Question. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) Above reaction is
(a) precipitation reaction
(b) double displacement reaction
(c) combination reaction
(d) Both (1) and (2)
Answer
4
Question. The correct formula of rust is
(a) Fe2O3
(b) Fe3O4
(c) Fe2O3.xH2O
(d) Fe3O4.xH2O
Answer
3
Question. Antioxidants are
(a) hydrating agents
(b) dehydrating agents
(c) oxidizing agents
(d) reducing agents
Answer
4
Question. Zn + (dil.) H2SO4 → ZnSO4 + H2↑ Above reaction is
(a) decomposition reaction.
(b) single displacement reaction.
(c) oxidation reaction.
(d) neutralisation reaction.
Answer
2
Question. When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is SO2 + 2H2S →2H2O + 3S. Here hydrogen sulphide is acting as
(a) an oxidising agent
(b) a reducing agent
(c) a dehydrating agent
(d) a catalyst
Answer
2
Question. CuO + H2 → H2O + Cu, reaction is an example of
(a) Redox reaction
(b) Decompostion reaction
(c) Neutralisation
(d) Analysis reaction
Answer
1
Question. Which of the statements about the following reactions is correct ?
ZnO + CO → Zn + CO2
(a) ZnO is being oxidized.
(b) CO is being reduced.
(c) CO2 is being oxidized.
(d) ZnO is being reduced.
Answer
4
Question. When a chemical species loses one or more electrons, it is said to have been
(a) oxidised
(b) reduced
(c) remain unchanged
(d) hydrolysed
Answer
1
Question. Which of the following is a redox reaction ?
(a) CaCO3 → CaO + CO2
(b) H2 + Cl2 → 2HCl
(c) CaO + 2HCl → CaCl2 + H2O
(d) NaOH + HCl → NaCl + H2O
Answer
2
Question. Which of the following statements is incorrect ?
(a) In oxidation, oxygen is added to a substance.
(b) In reduction, hydrogen is added to a substance.
(c) Oxidizing agent is oxidized.
(d) Reducing agent is oxidized.
Answer
3
Question. A substance which oxidises itself and reduces other is known as
(a) Oxidising agent
(b) Reducing agent
(c) Both of these
(d) None of these
Answer
2
Question. In the reaction PbO + C →Pb + CO
(a) PbO is oxidised.
(b) C acts as oxidising agent.
(c) C acts as reducing agent.
(d) This reaction does not represent redox reaction
Answer
2
Question. The process of oxidation involves
(a) the absorption of hydrogen atoms.
(b) the absorption of electrons.
(c) the release of electrons.
(d) neither absorption nor release of electrons.
Answer
3
Question. Rusting of an iron is an example of
(a) Reduction
(b) Ionization
(c) Oxidation
(d) Dissociation
Answer
3
Question. In the reaction 2MnO2 + 4Al →3Mn + 2Al2O3 the oxidising agent is
(a) MnO2
(b) Al
(c) Al2O3
(d) Mn
Answer
1
Question. Which of the following does not corrode when exposed to the atmosphere ?
(a) Iron
(b) Copper
(c) Gold
(d) Silver
Answer
3
Question. The oxidizing agent in the lead storage bettery is
(a) Pb
(b) PbO2
(c) PbSO4
(d) H2SO4
Answer
4
Question. When carbon dioxide gas is passed through lime water,
(a) calcium hydroxide is formed.
(b) white precipitate of CaO is formed.
(c) white precipitate of CaCO3 is formed.
(d) colour of lime water disappears.
Answer
3
Question. Zn2+(aq) + 2e– →Zn(s). This is
(a) Oxidation
(b) Reduction
(c) Redox reaction
(d) None of these
Answer
2
Question. The rate of a chemical reaction can be increased by adding a
(a) catalyst
(b) pigment
(c) reactant
(d) product
Answer
1
Question. Consider the reaction Zn + Cu2+ →Zn2+ + Cu With reference to the above, which one of the following is the correct statement ?
(a) Zn is reduced to Zn2+.
(b) Zn is oxidised to Zn2+.
(c) Zn2+ is oxidised to Zn.
(d) Cu2+ is oxidised to Cu.
Answer
2
Question. In the reaction PCl3 + Cl2 →PCl5
(a) PCl3 is acting as reductant.
(b) Cl2 is acting as reductant.
(c) Both PCl3 and Cl2 are acting as reductant.
(d) Both PCl3 and Cl2 are acting as oxidant.
Answer
1
Fill in the blanks
(a) The conversion of ferrous sulphate to ferric sulphate is ……………… reaction.
Answer
Oxidation
(b) Boiling of water to give water vapour is a ……………… change.
Answer
Physical
(c) The slow process of decay or destruction of a metal due to effect of air, moisture and acids on its own is known as ………………
Answer
Corrosion.
Match the following
Column I Column II
(a) BaCl2(aq) + ZnSO4(aq) → H2CO3(aq) (i) Displacement
(b) 2AgCl(s) → FeSO4(aq) + Cu(s) (ii) Combination
(c) CuSO4(aq) + Fe(s) → BaSO4↓ + ZnCl2(aq) (iii) Decomposition
(d) H2O(l) + CO2(g) → 2Ag(s) + Cl2(g) (iv) Double displacement
Answer
(a) (iv), (b) (iii), (c) (i), (d) (ii).
Assertion and Reasoning Based Questions
Directions : In the following questions, a statement of assertion is followed by a statement of reason. Mark the correct choice as :
(a) If both assertion and reason are true and reason is the correct explanation of assertion.
(b) If both assertion and reason are true, but reason is not the correct explanation of assertion.
(c) If assertion is true, but reason is false.
(d) If assertion is false but reason is true.
Question: Assertion : Hydrogen peroxide is kept in coloured bottles.
Reason : Hydrogen peroxide is a moderately reactive metal that can react with light or heat slowly to produce water.
Answer : (c) Hydrogen peroxide is a highly reactive metal that can react with light or heat to produce water. It decomposes into water and oxygen in the presence of sunlight. To prevent this reaction with light and heat it is stored in coloured bottles so that light cannot pass through it. Thus assertion is true, but reason is false.
Question: Assertion : Colour of copper sulphate solution changes when an iron nail is kept immersed in it.
Reason : The decomposition reaction takes place between iron and copper leading to the formation of iron sulphate.
Answer : (c) The colour of copper sulphate solution changes when iron nail is kept immersed in it due to the displacement reaction taking place between iron and copper leading to formation of iron sulphate. Thus assertion is true, but reason is false.
Question: Assertion : Chemical equations should be balanced.
Reason : As per the law of conservation of mass, mass can neither be created nor be destroyed.
Answer : (a) As per the law of conservation of mass — “mass can neither be created nor be destroyed”. Therefore number of atoms on both the sides of the equations should be equal. Thus both assertion and reason are true and reason is the correct explanation of the assertion.
Question: Assertion : Hydrogen is not included in the activity series of metals.
Reason : It is because it can lose electrons to form positive ions.
Answer : (d) Hydrogen is included in activity series of metals because like metals, hydrogen can also lose electrons to form positive ions. Thus assertion is false but reason is true.
Question: Assertion : Silver bromide is kept in the coloured bottles.
Reason : Silver bromide decomposes in presence of light.
Answer : (a) Silver bromide decomposes in presence of light, so as to prevent this decomposition; it is kept in coloured bottles. Thus both assertion and reason are true and reason is the correct explanation of the assertion.
Creating Based Questions
Question: 5ml of sulphuric acid was put into a small conical flask during some experiment in a laboratory. A small balloon has been fixed on the glass pipe inserted through the middle of the stopper cork. A student working in the lab adds some zinc granules to the conical flask by mistake and observes the balloon is blown up. Also, the conical flask became hot in the process.
(a) What may be the reason behind blowing up of balloon ?
(b) What may be the chemical representation of the above observation ?
Answer : (a) The zinc granules undergo a chemical reaction with sulphuric acid to give hydrogen gas as a product.
This evolved hydrogen gas fills the balloon and balloon gets blown up.
(b) As this is a chemical reaction, this can be represented by the following chemical equation :
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Question: Give reason and name the type of chemical reaction taking place in each case :
(a) Dissolution of ammonium chloride in water leads to cooling of the glass apparatus used for dissolutions.
(b) Silver chloride powder which is white in colour, turns grey when kept in sunlight.
(c) Blue colour of copper sulphate solution fades when an iron nail is dipped inside the solution.
Answer : (a) Dissolution of ammonium chloride in water is an endothermic reaction where heat is absorbed from surroundings hence making the surrounding cooler than before :
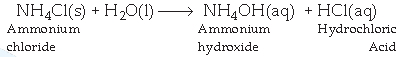
(b) Silver chloride undergoes decomposition reaction in sunlight to give silver metal and chlorine :
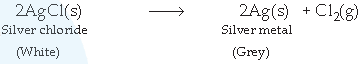
(c) Iron displaces copper from its solution, hence the colour of copper sulphate solution becomes less blue or fades. This is an example of displacement reaction

Question: Annie was happy to see her small house getting cleaned and whitewashed. She observed that the workers dissolved some pale white powdery material called quick lime into water (a vigorous reaction takes place while mixing) left it for some time and applied the white suspension obtained to the walls. At first the wall looked pale white and watery but in 2-3 days the walls became shiny bright white in colour. How can you describe what she observed through chemical equations ?
Answer : Quick lime is chemically known as calcium oxide. It reacts vigorously with water to produce slaked lime (calcium hydroxide), releasing a large amount of heat.
CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat
Quick lime Slaked lime
The slaked lime after application to wall reacts slowly with carbon dioxide present in atmosphere, to give a thin layer of calcium carbonate on the walls after 2-3 days giving the walls a shiny bright white colour.
Question: Write a balanced chemical equation with state symbols for the following reactions :
(a) Glucose molecule (C6H12O6) reacts with oxygen molecule to give carbon dioxide gas and gaseous water.
(b) Iron dust reacts with gaseous water to give iron oxide (Fe3O4) precipitate and hydrogen gas.
Answer : (a) C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O
(b) 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Ca(OH)2(aq) + CO2(g) → CaCO3 + H2O(l)
Slaked lime Calcium
carbonate
Question: Which species out of (1) and (2) is getting oxidised and which one is getting reduced in the below given reactions and why :

Answer :
(a) Here cuprous oxide (1) is getting reduced (to give copper metal) as it is losing oxygen and hydrogen
(2) is getting oxidised as it is combining with oxygen to give water.
(b) Here manganese dioxide (1) is getting reduced (to MnCl2) as it is losing oxygen and hydrochloric acid
(2) is getting oxidised to water.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Manganese Hydrochloric
dioxide acid
Generally, these type of reactions are known as redox reaction.
Paragraph/Table and Case Study Based Questions
1. Read the following and answer any four questions from 1(i) to 1(v):
Vedanshi and Krati are working in the lab. During the experiment of determining the reactivity of acid and bases, the test tube containing chemical ‘X’ cracked and the liquid inside produced blisters on the skin of the Krati. The pH strip turned blue to red, with the reaction to the liquid. Vedanshi immediately called her teacher for help, and first aid was provided to Krati.
(i) What could be the possible liquid be present in test tube?
(a) Boiling water
(b) Concentrated H2SO4
(c) Vinegar
(d) Sodium Hydroxide Solution
Answer
B
(ii) The following list include the possible first aid for the laboratory accidents.
1. Thoroughly rinse the affected area with water.
1. Thoroughly rinse the affected area with water.
2. Having medicines without prescribing the doctor.
3. Consult the doctor immediately
4. To go outside the lab, preferably in an open area.
5. Not telling the teacher and other elders or they might scold you.
(a) All of these
(b) 1, 2 and 3 are correct
(c) Only 1 is correct
(d) 1, 3 and 4 are correct
Answer
D
(iii) The given graph below is plotted again pH and H+ concentration.

In the experiment, Krati and Vedanshi listed the chemicals in list A and B. Which of the following is correct?
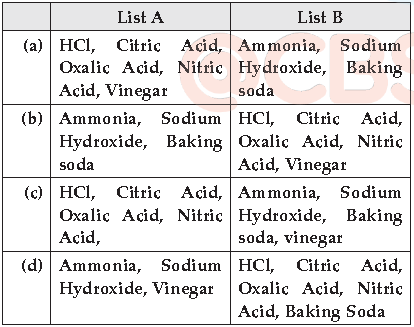
Answer
B
(iv) Krati detected the pH of four unknown solution A, B, C and D as follows 11, 5, 7 and 2 respectively and note down her results.
1. C is neutral in nature.
2. On reacting A and D, water is liberated.
3. A and C are basic in nature.
4. B and D react to form hydrogen gas
(a) All are correct
(b) 1 and 2 are correct
(c) 1 and 4 are correct
(d) 1, 2 and 3 are correct.
Answer
B
(v) What does the following activity represent?
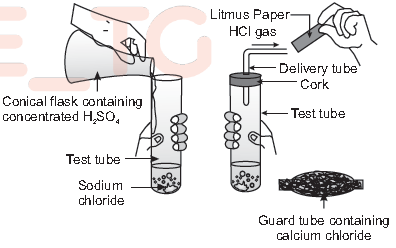
(a) Production of HCl
(b) Production of H2SO4
(c) Production of CO2
(d) Reaction of calcium chloride with sulphuric acid.
Answer
A
2. Read the following and answer any four questions from 20 (i) to 20 (v): Balanced chemical equation has an equal number of atoms of different elements in the reactants and products. According to law of conservation of mass, matter can neither be created nor be destroyed in a chemical reaction.
(i) Which of the following equation are balanced?
(a) 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(b) 2CaO(s) + H2O(l) → 2Ca(OH)2(aq)
(c) CH4(g) + 2O2(g) → CO2 (g) + 2H2O(g)
(d) 2AgBr(s) → Ag(s) + 2Br2(g)
Answer
A
(ii) The following represent the exothermic reaction. The reactants react to from product, with the release of energy in the from of heat.
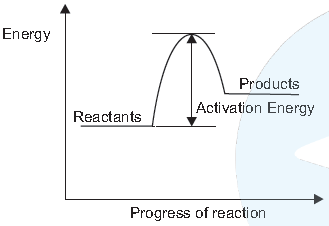
Which of the following involve an exothemic reaction?
(a) Respiration
(b) Oxidation
(c) Combustion
(d) All of these
Answer
D
(iii) When the powder of a common metal is heated in an open china dish, its colour turns black.
However, when hydrogen is passed over the hot black substance so formed, it regains its original colour. What type of chemical reaction takes place in each of the two given steps?
(a) First oxidation reaction then displacement reaction
(b) First combustion reaction, then reduction reaction
(c) First oxidation reaction, then redox reaction
(d) First reduction reaction, then redox reaction
Answer
C
(iv) Study the given diagram in which white powder of silver chloride was left open in sunlight. After few hours, it was observed that the white powder changed to silvery material. What type of reaction is it?
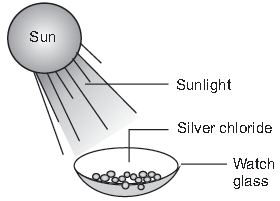
(a) Displacement reaction
(b) Decomposition reaction
(c) Redox reaction
(d) Corrosion
Answer
B
(v) After rain, Shreya observed that the iron lock of the main gate of her house had a reddish-brown powdery covering. The cleaned the lock using sandpaper and found the shiny, non-corroded layer beneath. What could she do to prevent such reaction to occur?

(a) To buy a new similar lock.
(b) To coat the surface with a layer of paint.
(c) To clean the lock using sandpaper regularly.
(d) To buy a gold lock instead of iron lock.
Answer
B









